With an intense focus on quality, medical device manufacturers require an automation equipment provider who is intimately familiar with the challenges they face an can accommodate the timeline and needs of their production teams — or, at least they should. That’s because maximizing on the potential of automation in the medical device industry requires a unique approach that offers support throughout the entire project. In this eBook, we share the stories of four customers — all from segments within the … Read More
Medical Device Manufacturers are Improving OEE with Automation
What is OEE? OEE – Overall Equipment Effectiveness – measures how well a manufacturing operation is performing compared to its full potential. To calculate OEE, we identify the availability, performance, and quality of the manufacturing process.
Automated End-of-Line Tester Adds RFID Tagging
How Invotec designed an automated end-of-line tester that helped one medical device manufacturer scale-up production while protecting IP The Challenge A large manufacturer of pathology solutions faced increased demands for their tissue-sampling probes. They forecasted volumes rising nearly 400% over the next five years—and they needed an additional, automated end-of-line tester to meet those goals. The company also wanted to add RFID tagging to protect their intellectual property as their products began to be sold internationally. The Solution Having designed … Read More
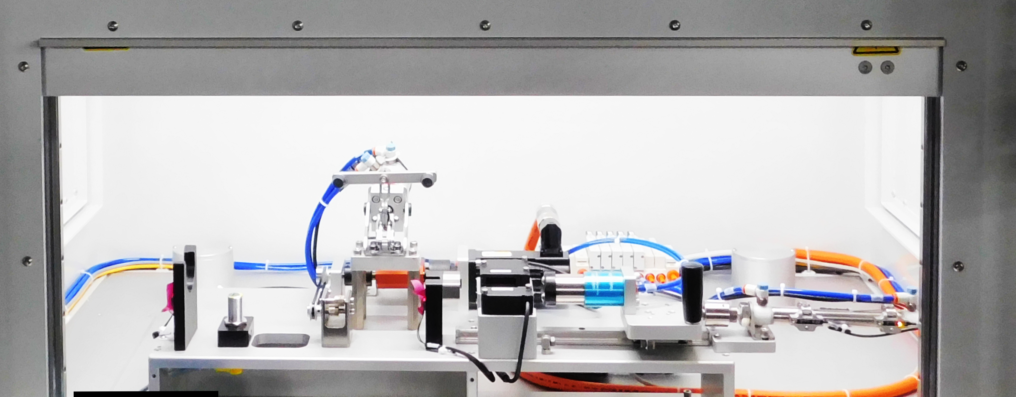
Tech Tip: Test Systems & Anticipating Your Unknowns
Solid test systems are a critical component in medical device manufacturing—and for good reason. High international standards set by the FDA and CE regulatory bodies help keep patients and healthcare professionals safe, making compliance necessary but not always easy to achieve. Robust test equipment helps manufacturers ensure that devices can withstand external forces not tested during the assembly process. Designing these systems always presents the same challenge: engineers must learn to predict what they don’t know. So how do they … Read More